SEARCH
検索詳細木村 行宏大学院農学研究科 生命機能科学専攻准教授
研究者基本情報
■ 学位■ 研究ニュース
■ 研究キーワード
■ 研究分野
研究活動情報
■ 論文- American Chemical Society (ACS), 2025年06月, Biochemistry[査読有り]研究論文(学術雑誌)
- American Chemical Society (ACS), 2025年06月, The Journal of Physical Chemistry Letters, 16(25) (25), 6364 - 6371[査読有り]研究論文(学術雑誌)
- Abstract BACKGROUND Lactobacillus panisapium, isolated from the gastrointestinal tract of a queen honeybee, converts trans‐10‐hydroxy‐2‐decenoic acid (10H2DA) into 10‐hydroxydecanoic acid (10HDAA) in royal jelly. 10HDAA has shown significant anti‐inflammatory potential. This study aimed to identify the reductase responsible for this bioconversion and characterize its properties. RESULTS An enzyme was partially purified from L. panisapium cell extracts based on its 10H2DA‐reducing activity. Liquid chromatography–mass spectrometry and phylogenetic analyses identified the enzyme as a water‐soluble fumarate reductase (~40% identity to reductases from Shewanella frigidimarina and Klebsiella pneumoniae). The reductase was heterologously expressed in Escherichia coli. The recombinant enzyme displayed activity toward fumarate and 10H2DA in the presence of flavin adenine dinucleotide and nicotinamide adenine dinucleotide hydride. The enzyme exhibited optimal stability at 50 °C and pH 6.0. An active‐site glycine residue in the L. panisapium reductase likely facilitates the binding of large substrates such as 10H2DA. Substituting this residue with threonine decreased activity toward 10H2DA while maintaining activity toward fumarate. Structural modeling revealed that, compared with the homologous reductase from S. frigidimarina, the enzyme from L. panisapium lacks a heme‐binding domain, again consistent with enhanced access for larger substrates. Whole cells of E. coli expressing the recombinant reductase effectively converted 10H2DA to 10HDAA, offering a scalable alternative to using purified recombinant enzyme. CONCLUSION This study provides insights into the structure–function relationship of a novel fumarate reductase‐family enzyme from L. panisapium and demonstrates its potential for biocatalytic applications in producing 10HDAA‐enriched royal jelly, contributing to the development of functional foods. © 2025 Society of Chemical Industry.Wiley, 2025年06月, Journal of the Science of Food and Agriculture[査読有り]研究論文(学術雑誌)
- Abstract Background Fermented katsuobushi, a traditional Japanese seasoning, is produced from skipjack tuna through smoking, drying and fermentation by xerophilic Aspergillus molds, primarily Aspergillus chevalieri and Aspergillus pseudoglaucus. In this study, we characterized lipolytic enzymes (cLip_1 to cLip_5 and pLip_1 to pLip_3) to clarify their roles in lipid hydrolysis during katsuobushi production under low water activity. Results The enzymes showed significant diversity in their activity, stability and substrate specificity, and in the hydrolysis profiles of their reactions with fish oil. Phylogenetic analyses revealed that cLip_5 showed a high identity with pLip_2 (94%) and these enzymes formed a phylogenetic cluster with filamentous fungal lipases. Purified recombinant enzymes (rcLip_1, rcLip_2, rcLip_4 and rcLip_5) and wild‐type enzymes (cLip_3 and pLip_3) showed varying substrate preferences toward p‐nitrophenyl esters. The addition of glycerol to reduce the water activity in the reaction mixture led to increased activities of rcLip_1 and rcLip_4, but it did not affect the activity of the other three enzymes. Among the tested six enzymes, cLip_5 showed the highest hydrolytic activity toward fish oil. The cLip_5 and pLip_2 gene transcript levels were moderately high in strains MK86 and MK88, respectively. Conclusion cLip_5 and its homolog pLip_2 were identified as the most promising enzymes for katsuobushi fermentation, because of their high hydrolytic activities toward fish oil and adaptability to low water activity conditions. These findings support the selection of optimal Aspergillus strains as starter cultures to potentially shorten the fermentation time and improve the quality and shelf life of katsuobushi. © 2025 Society of Chemical Industry.Wiley, 2025年02月, Journal of the Science of Food and Agriculture, 英語[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2025年02月, Nature Communications, 16(1) (1), 英語[査読有り]研究論文(学術雑誌)
- Abstract Rhodothalassium (Rts.) salexigens is a halophilic purple nonsulfur bacterium and the sole species in the genus Rhodothalassium, which is itself the sole genus in the family Rhodothalassiaceae and sole family in the order Rhodothalassiales (class Alphaproteobacteria). The genome of this phylogenetically unique phototroph comprises 3.35 Mb and is highly chimeric, with nearly half of its genes originating from families other than the Rhodothalassiaceae, many of which lack phototrophic species. Photosynthesis genes in Rts. salexigens are not arranged in a typical photosynthesis gene cluster but are scattered across the genome, suggesting an origin from horizontal transfers. Despite an encoded RuBisCO, autotrophy has not been observed in Rts. salexigens, and enzymes that oxidize common inorganic electron donors are not encoded. Phospholipid biosynthesis in Rts. salexigens is restricted, and phosphoglycerolipids are the only phospholipids present in its intracytoplasmic membranes. Rts. salexigens fixes nitrogen using a Mo-containing nitrogenase and uses ammonia despite previous results that indicated it was a glutamate auxotroph. Glycine betaine is the sole osmolyte in Rts. salexigens, and enzymes are encoded that facilitate both its uptake and its biosynthesis from glycine. The genomic data also support chemotactic swimming motility, growth over a range of salinities, and the production of membrane-strengthening hopanoids.Springer Science and Business Media LLC, 2025年01月, Extremophiles, 29(1) (1)[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2025年01月, Communications Biology, 8(1) (1), 英語[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2024年12月, Communications Biology, 7(1) (1)[査読有り]研究論文(学術雑誌)
- American Chemical Society (ACS), 2024年12月, Biochemistry, 64(1) (1), 170 - 179, 英語[査読有り]研究論文(学術雑誌)
- Elsevier BV, 2024年08月, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 149503 - 149503, 英語[査読有り]研究論文(学術雑誌)
- ABSTRACT Halorhodospira (Hlr.) halochloris is a triply extremophilic phototrophic purple sulfur bacterium, as it is thermophilic, alkaliphilic, and extremely halophilic. The light‐harvesting‐reaction center (LH1–RC) core complex of this bacterium displays an LH1‐Qy transition at 1,016 nm, which is the lowest‐energy wavelength absorption among all known phototrophs. Here we report the cryo‐EM structure of the LH1–RC at 2.42 Å resolution. The LH1 complex forms a tricyclic ring structure composed of 16 αβγ‐polypeptides and one αβ‐heterodimer around the RC. From the cryo‐EM density map, two previously unrecognized integral membrane proteins, referred to as protein G and protein Q, were identified. Both of these proteins are single transmembrane‐spanning helices located between the LH1 ring and the RC L‐subunit and are absent from the LH1–RC complexes of all other purple bacteria of which the structures have been determined so far. Besides bacteriochlorophyll b molecules (B1020) located on the periplasmic side of the Hlr. halochloris membrane, there are also two arrays of bacteriochlorophyll b molecules (B800 and B820) located on the cytoplasmic side. Only a single copy of a carotenoid (lycopene) was resolved in the Hlr. halochloris LH1–α3β3 and this was positioned within the complex. The potential quinone channel should be the space between the LH1–α3β3 that accommodates the single lycopene but does not contain a γ‐polypeptide, B800 and B820. Our results provide a structural explanation for the unusual Qy red shift and carotenoid absorption in the Hlr. halochloris spectrum and reveal new insights into photosynthetic mechanisms employed by a species that thrives under the harshest conditions of any phototrophic microorganism known.Wiley, 2024年02月, Journal of Integrative Plant Biology, 英語[査読有り]研究論文(学術雑誌)
- Abstract The ecological success of purple sulfur bacteria (PSB) is linked to their ability to collect near‐infrared solar energy by membrane‐integrated, pigment–protein photocomplexes. These include a Core complex containing both light‐harvesting 1 (LH1) and reaction centre (RC) components (called the LH1–RC photocomplex) present in all PSB and a peripheral light‐harvesting complex present in most but not all PSB. In research to explain the unusual absorption properties of the thermophilic purple sulfur bacterium Thermochromatium tepidum, Ca2+ was discovered bound to LH1 polypeptides in its LH1–RC; further work showed that calcium controls both the thermostability and unusual spectrum of the Core complex. Since then, Ca2+ has been found in the LH1–RC photocomplexes of several other PSB, including mesophilic species, but not in the LH1–RC of purple non‐sulfur bacteria. Here we focus on four species of PSB—two thermophilic and two mesophilic—and describe how Ca2+ is integrated into and affects their photosynthetic machinery and why this previously overlooked divalent metal is a key nutrient for their ecological success.Wiley, 2024年02月, Environmental Microbiology, 26(2) (2), 英語[査読有り][招待有り]研究論文(学術雑誌)
- Abstract The mesophilic purple sulfur phototrophic bacterium Allochromatium (Alc.) vinosum (bacterial family Chromatiaceae) has been a favored model for studies of bacterial photosynthesis and sulfur metabolism, and its core light-harvesting (LH1) complex has been a focus of numerous studies of photosynthetic light reactions. However, despite intense efforts, no high-resolution structure and thorough biochemical analysis of the Alc. vinosum LH1 complex have been reported. Here we present cryo-EM structures of the Alc. vinosum LH1 complex associated with reaction center (RC) at 2.24 Å resolution. The overall structure of the Alc. vinosum LH1 resembles that of its moderately thermophilic relative Alc. tepidum in that it contains multiple pigment-binding α- and β-polypeptides. Unexpectedly, however, six Ca ions were identified in the Alc. vinosum LH1 bound to certain α1/β1- or α1/β3-polypeptides through a different Ca2+-binding motif from that seen in Alc. tepidum and other Chromatiaceae that contain Ca2+-bound LH1 complexes. Two water molecules were identified as additional Ca2+-coordinating ligands. Based on these results, we reexamined biochemical and spectroscopic properties of the Alc. vinosum LH1–RC. While modest but distinct effects of Ca2+ were detected in the absorption spectrum of the Alc. vinosum LH1 complex, a marked decrease in thermostability of its LH1–RC complex was observed upon removal of Ca2+. The presence of Ca2+ in the photocomplex of Alc. vinosum suggests that Ca2+-binding to LH1 complexes may be a common adaptation in species of Chromatiaceae for conferring spectral and thermal flexibility on this key component of their photosynthetic machinery.Springer Science and Business Media LLC, 2024年02月, Communications Biology, 7(1) (1), 英語[査読有り]研究論文(学術雑誌)
- Elsevier BV, 2023年11月, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1864(4) (4), 149001 - 149001, 英語[査読有り]研究論文(学術雑誌)
- 2023年09月, ARCHIVES OF MICROBIOLOGY, 205(9) (9), 英語[査読有り]研究論文(学術雑誌)
- 2023年08月, EXTREMOPHILES, 27(2) (2), 英語[査読有り]研究論文(学術雑誌)
- 2023年07月, PROCESS BIOCHEMISTRY, 130, 534 - 544, 英語[査読有り]研究論文(学術雑誌)
- Elsevier BV, 2023年04月, Enzyme and Microbial Technology, 110240 - 110240, 英語[査読有り]研究論文(学術雑誌)
- Structural information on the circular arrangements of repeating pigment-polypeptide subunits in antenna proteins of purple photosynthetic bacteria is a clue to a better understanding of molecular mechanisms for the ring-structure formation and efficient light harvesting of such antennas. Here, we have analyzed the ring structure of light-harvesting complex 2 (LH2) from the thermophilic purple bacterium Thermochromatium tepidum (tepidum-LH2) by atomic force microscopy. The circular arrangement of the tepidum-LH2 subunits was successfully visualized in a lipid bilayer. The average top-to-top distance of the ring structure, which is correlated with the ring size, was 4.8 ± 0.3 nm. This value was close to the top-to-top distance of the octameric LH2 from Phaeospirillum molischianum (molischianum-LH2) by the previous analysis. Gaussian distribution of the angles of the segments consisting of neighboring subunits in the ring structures of tepidum-LH2 yielded a median of 44°, which corresponds to the angle for the octameric circular arrangement (45°). These results indicate that tepidum-LH2 has a ring structure consisting of eight repeating subunits. The coincidence of an octameric ring structure of tepidum-LH2 with that of molischianum-LH2 is consistent with the homology of amino acid sequences of the polypeptides between tepidum-LH2 and molischianum-LH2.2023年03月, Photosynthesis research, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- Rhodobacter (Rba.) capsulatus has been a favored model for studies of all aspects of bacterial photosynthesis. This purple phototroph contains PufX, a polypeptide crucial for dimerization of the light-harvesting 1-reaction center (LH1-RC) complex, but lacks protein-U, a U-shaped polypeptide in the LH1-RC of its close relative Rba. sphaeroides. Here we present a cryo-EM structure of the Rba. capsulatus LH1-RC purified by DEAE chromatography. The crescent-shaped LH1-RC exhibits a compact structure containing only 10 LH1 αβ-subunits. Four αβ-subunits corresponding to those adjacent to protein-U in Rba. sphaeroides were absent. PufX in Rba. capsulatus exhibits a unique conformation in its N-terminus that self-associates with amino acids in its own transmembrane domain and interacts with nearby polypeptides, preventing it from interacting with proteins in other complexes and forming dimeric structures. These features are discussed in relation to the minimal requirements for the formation of LH1-RC monomers and dimers, the spectroscopic behavior of both the LH1 and RC, and the bioenergetics of energy transfer from LH1 to the RC.2023年02月, Nature communications, 14(1) (1), 846 - 846, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- American Chemical Society (ACS), 2023年01月, The Journal of Physical Chemistry B, 英語[査読有り][招待有り]研究論文(学術雑誌)
- Abstract Rhodobacter sphaeroides is a model organism in bacterial photosynthesis, and its light-harvesting-reaction center (LH1–RC) complex contains both dimeric and monomeric forms. Here we present cryo-EM structures of the native LH1–RC dimer and an LH1–RC monomer lacking protein-U (ΔU). The native dimer reveals several asymmetric features including the arrangement of its two monomeric components, the structural integrity of protein-U, the overall organization of LH1, and rigidities of the proteins and pigments. PufX plays a critical role in connecting the two monomers in a dimer, with one PufX interacting at its N-terminus with another PufX and an LH1 β-polypeptide in the other monomer. One protein-U was only partially resolved in the dimeric structure, signaling different degrees of disorder in the two monomers. The ΔU LH1–RC monomer was half-moon-shaped and contained 11 α- and 10 β-polypeptides, indicating a critical role for protein-U in controlling the number of αβ-subunits required for dimer assembly and stabilization. These features are discussed in relation to membrane topology and an assembly model proposed for the native dimeric complex.Springer Science and Business Media LLC, 2022年12月, Nature Communications, 13(1) (1), 1904 - 1904, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- Abstract Rhodopila globiformis is the most acidophilic of anaerobic purple phototrophs, growing optimally in culture at pH 5. Here we present a cryo-EM structure of the light-harvesting 1–reaction center (LH1–RC) complex from Rhodopila globiformis at 2.24 Å resolution. All purple bacterial cytochrome (Cyt, encoded by the gene pufC) subunit-associated RCs with known structures have their N-termini truncated. By contrast, the Rhodopila globiformis RC contains a full-length tetra-heme Cyt with its N-terminus embedded in the membrane forming an α-helix as the membrane anchor. Comparison of the N-terminal regions of the Cyt with PufX polypeptides widely distributed in Rhodobacter species reveals significant structural similarities, supporting a longstanding hypothesis that PufX is phylogenetically related to the N-terminus of the RC-bound Cyt subunit and that a common ancestor of phototrophic Proteobacteria contained a full-length tetra-heme Cyt subunit that evolved independently through partial deletions of its pufC gene. Eleven copies of a novel γ-like polypeptide were also identified in the bacteriochlorophyll a-containing Rhodopila globiformis LH1 complex; γ-polypeptides have previously been found only in the LH1 of bacteriochlorophyll b-containing species. These features are discussed in relation to their predicted functions of stabilizing the LH1 structure and regulating quinone transport under the warm acidic conditions.Springer Science and Business Media LLC, 2022年11月, Communications Biology, 5(1) (1)[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2022年10月, 3 Biotech, 12(10) (10)[査読有り]研究論文(学術雑誌)
- Halorhodospira (Hlr.) species are the most halophilic and alkaliphilic of all purple bacteria. Hlr. halochloris exhibits the lowest LH1 Qy transition energy among phototrophic organisms and is the only known triply extremophilic anoxygenic phototroph, displaying a thermophilic, halophilic, and alkaliphilic phenotype. Recently, we reported that electrostatic charges are responsible for the unusual spectroscopic properties of the Hlr. halochloris LH1 complex. In the present work, we examined the effects of salt and pH on the spectroscopic properties and thermal stability of LH1-RCs from Hlr. halochloris compared with its mesophilic counterpart, Hlr. abdelmalekii. Experiments in which the photocomplexes were subjected to different levels of salt or variable pH revealed that the thermal stability of LH1-RCs from both species was largely retained in the presence of high salt concentrations and/or at alkaline pH but was markedly reduced by lowering the salt concentration and/or pH. Based on the amino acid sequences of LH1 polypeptides and their composition of acidic/basic residues and the Hofmeister series for cation/anion species, we discuss the importance of electrostatic charge in stabilizing the Hlr. halochloris LH1-RC complex to allow it to perform photosynthesis in its warm, hypersaline, and alkaline habitat.MDPI AG, 2022年05月, Microorganisms, 10(5) (5), 959 - 959, 英語[査読有り][招待有り]研究論文(学術雑誌)
- The mildly thermophilic purple phototrophic bacterium Allochromatium tepidum provides a unique model for investigating various intermediate phenotypes observed between those of thermophilic and mesophilic counterparts. The core light-harvesting (LH1) complex from A. tepidum exhibits an absorption maximum at 890 nm and mildly enhanced thermostability, both of which are Ca2+-dependent. However, it is unknown what structural determinants might contribute to these properties. Here, we present a cryo-EM structure of the reaction center-associated LH1 complex at 2.81 Å resolution, in which we identify multiple pigment-binding α- and β-polypeptides within an LH1 ring. Of the 16 α-polypeptides, we show that six (α1) bind Ca2+ along with β1- or β3-polypeptides to form the Ca2+-binding sites. This structure differs from that of fully Ca2+-bound LH1 from Thermochromatium tepidum, enabling determination of the minimum structural requirements for Ca2+-binding. We also identified three amino acids (Trp44, Asp47, and Ile49) in the C-terminal region of the A. tepidum α1-polypeptide that ligate each Ca ion, forming a Ca2+-binding WxxDxI motif that is conserved in all Ca2+-bound LH1 α-polypeptides from other species with reported structures. The partial Ca2+-bound structure further explains the unusual phenotypic properties observed for this bacterium in terms of its Ca2+-requirements for thermostability, spectroscopy, and phototrophic growth, and supports the hypothesis that A. tepidum may represent a "transitional" species between mesophilic and thermophilic purple sulfur bacteria. The characteristic arrangement of multiple αβ-polypeptides also suggests a mechanism of molecular recognition in the expression and/or assembly of the LH1 complex that could be regulated through interactions with reaction center subunits.Elsevier BV, 2022年04月, Journal of Biological Chemistry, 298(6) (6), 101967 - 101967, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- AIP Publishing, 2022年02月, The Journal of Chemical Physics[査読有り][招待有り]研究論文(学術雑誌)
- Wiley, 2022年02月, Journal of Basic Microbiology, 62(2) (2), 174 - 184[査読有り]研究論文(学術雑誌)
- 2022年01月, Photochemistry and Photobiology, 98(1) (1), 169 - 174, 英語[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2021年12月, Nature Communications, 12(1) (1), 6300 - 6300, 英語, 国際誌
Abstract Rhodobacter (Rba .)sphaeroides is the most widely used model organism in bacterial photosynthesis. The light-harvesting-reaction center (LH1-RC) core complex of this purple phototroph is characterized by the co-existence of monomeric and dimeric forms, the presence of the protein PufX, and approximately two carotenoids per LH1 αβ-polypeptides. Despite many efforts, structures of theRba. sphaeroides LH1-RC have not been obtained at high resolutions. Here we report a cryo-EM structure of the monomeric LH1-RC fromRba. sphaeroides strain IL106 at 2.9 Å resolution. The LH1 complex forms a C-shaped structure composed of 14 αβ-polypeptides around the RC with a large ring opening. From the cryo-EM density map, a previously unrecognized integral membrane protein, referred to as protein-U, was identified. Protein-U has a U-shaped conformation near the LH1-ring opening and was annotated as a hypothetical protein in theRba. sphaeroides genome. Deletion of protein-U resulted in a mutant strain that expressed a much-reduced amount of the dimeric LH1-RC, indicating an important role for protein-U in dimerization of the LH1-RC complex. PufX was located opposite protein-U on the LH1-ring opening, and both its position and conformation differed from that of previous reports of dimeric LH1-RC structures obtained at low-resolution. Twenty-six molecules of the carotenoid spheroidene arranged in two distinct configurations were resolved in theRba. sphaeroides LH1 and were positioned within the complex to block its channels. Our findings offer an exciting new view of the core photocomplex ofRba. sphaeroides and the connections between structure and function in bacterial photocomplexes in general.[査読有り]研究論文(学術雑誌) - 2021年12月, Nature Communications, 12(1) (1), 英語[査読有り]研究論文(学術雑誌)
- 2021年11月, BIOCHIMICA ET BIOPHYSICA ACTA-BIOENERGETICS, 1862(11) (11), 英語[査読有り]研究論文(学術雑誌)
- Elsevier BV, 2021年09月, International Journal of Food Microbiology, 353, 109299 - 109299[査読有り]研究論文(学術雑誌)
- 2021年09月, BIOCHEMISTRY, 60(36) (36), 2685 - 2690, 英語[査読有り]研究論文(学術雑誌)
- 2021年08月, BIOCHEMISTRY, 60(32) (32), 2483 - 2491, 英語[査読有り]研究論文(学術雑誌)
- Cold Spring Harbor Laboratory, 2021年06月, 英語
Abstract We present a cryo-EM structure of the monomeric light-harvesting-reaction center (LH1-RC) core complex from photosynthetic purple bacteriumRhodobacter (Rba .)sphaeroides at 2.9 Å resolution. The LH1 complex forms a C-shaped structure composed of 14 αβ-polypeptides around the RC with a large ring opening. From the cryo-EM density map, a previously unrecognized integral membrane protein, referred to as protein-U, was identified. Protein-U has a U-shaped conformation near the LH1-ring opening and was annotated as a hypothetical protein in theRba. sphaeroides genome. Deletion of protein-U resulted in a mutant strain that expressed a much-reduced amount of the dimeric LH1-RC, indicating an important role for protein-U in dimerization of the LH1-RC complex. PufX was located opposite protein-U on the LH1-ring opening, and both its position and conformation differed from that of previous reports of dimeric LH1-RC structures obtained at low-resolution. Twenty-six molecules of the carotenoid spheroidene arranged in two distinct configurations were resolved in theRba. sphaeroides LH1 and were positioned within the complex to block its pores. Our findings offer a new view of the core photocomplex ofRba. sphaeroides and the connections between structure and function in bacterial photocomplexes in general.研究論文(学術雑誌) - Cold Spring Harbor Laboratory, 2021年05月
Abstract We present a cryo-EM structure of the light-harvesting-reaction center (LH1-RC) core complex from purple phototrophic bacteriumRhodospirillum (Rsp .)rubrum at 2.76 Å resolution. The LH1 complex forms a closed, slightly elliptical ring structure with 16 αβ-polypeptides surrounding the RC. Our biochemical analysis detected rhodoquinone (RQ) molecules in the purified LH1-RC, and the cryo-EM density map specifically positions RQ at the QA site in the RC. The geranylgeraniol sidechains of bacteriochlorophyll (BChl)a G coordinated by LH1 β-polypeptides exhibit a highly homologous tail-up conformation that allows for interactions with the bacteriochlorin rings of nearby LH1 α-associated BChlsa G. The structure also revealed key protein–protein interactions in both N- and C-terminal regions of the LH1 αβ-polypeptides, mainly within a face-to-face structural subunit. Our findings enable to evaluate past experimental and computational results obtained with this widely used organism and provide crucial information for more detailed exploration of light-energy conversion, quinone transport, and structure–function relationships in pigment-protein complexes. - Springer Science and Business Media LLC, 2021年05月, Photosynthesis Research, 148(1-2) (1-2), 77 - 86[査読有り]研究論文(学術雑誌)
- 2021年03月, The Journal of Physical Chemistry B, 125(8) (8), 2009 - 2017, 英語[査読有り]研究論文(学術雑誌)
- 2021年01月, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1862(1) (1), 148307 - 148307, 英語[査読有り]研究論文(学術雑誌)
- Springer Science and Business Media LLC, 2020年12月, Nature Communications, 11(1) (1), 4955 - 4955, 英語, 国際誌
Abstract The light-harvesting-reaction center complex (LH1-RC) from the purple phototrophic bacteriumThiorhodovibrio strain 970 exhibits an LH1 absorption maximum at 960 nm, the most red-shifted absorption for any bacteriochlorophyll (BChl)a -containing species. Here we present a cryo-EM structure of the strain 970 LH1-RC complex at 2.82 Å resolution. The LH1 forms a closed ring structure composed of sixteen pairs of the αβ-polypeptides. Sixteen Ca ions are present in the LH1 C-terminal domain and are coordinated by residues from the αβ-polypeptides that are hydrogen-bonded to BChla . The Ca2+-facilitated hydrogen-bonding network forms the structural basis of the unusual LH1 redshift. The structure also revealed the arrangement of multiple forms of α- and β-polypeptides in an individual LH1 ring. Such organization indicates a mechanism of interplay between the expression and assembly of the LH1 complex that is regulated through interactions with the RC subunits inside.[査読有り]研究論文(学術雑誌) - A soup stock made from katsuobushi is an important element of, and the basic seasoning responsible for the taste of, traditional Japanese cuisine. Fermented and ripened katsuobushi, called karebushi, is manufactured via a repeated molding process on the katsuobushi surface. Our aim was to characterize the surface Aspergillus community and their enzymes involved in the fermentation and ripening. Five dominant Aspergillus species isolated from the karebushi surface were identified-A. amstelodami, A. chevalieri, A. pseudoglaucus, A. ruber, and A. sydowii. Analyses were performed on final molding stage-samples from different manufacturers, and 1st to 4th molding stage-samples from the same manufacturer. The composition ratios of the five Aspergillus spp. varied according to the manufacturer of the karebushi. A. amstelodami and A. chevalieri tended to be detected as dominant species when the water content of the karebushi fillet was >15% and the fat content was >3.5%, respectively. In samples from a given manufacturer, the dominant species in the final molding stage tended to be A. chevalieri and A. pseudoglaucus. Mixed molds were cultured by solid-state fermentation using katsuobushi powder medium at two different water activity (aw) levels. Crude extracts from each culture showed lipase, aminopeptidase, carboxypeptidase, and protease activities. Notably, the crude extracts cultivated at 0.85 aw showed higher protease activity toward hemoglobin and lipase activity toward p-nitrophenyl palmitate than those at 0.95 aw. These hydrolytic enzymes are probably involved in decolorization of katsuobushi and lipid degradation during the long fermentative and ripening period. In addition, mixed cultures could transform 2,6-dimethoxyphenol into 1,2,3-trimethoxybenzene, previously reported as an attractive and mild flavor component. Our results may help promote the use of desirable Aspergillus spp. as starter cultures for manufacturers to stabilize and improve the quality of fermented and ripened karebushi.Elsevier BV, 2020年08月, International Journal of Food Microbiology, 327, 108654 - 108654, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- 2020年06月, BIOCHEMISTRY, 59(25) (25), 2351 - 2358, 英語[査読有り]研究論文(学術雑誌)
- 2020年03月, ACS OMEGA, 5(12) (12), 6817 - 6825, 英語[査読有り]研究論文(学術雑誌)
- 2019年10月, PROCESS BIOCHEMISTRY, 85, 156 - 163[査読有り]研究論文(学術雑誌)
- 2019年06月, BIOCHEMISTRY, 58(25) (25), 2844 - 2852, 英語[査読有り]研究論文(学術雑誌)
- 2019年04月, PROCESS BIOCHEMISTRY, 79, 74 - 80[査読有り]研究論文(学術雑誌)
- 2019年04月, Biochimica Biophysica Acta, 1860, 461 - 468Phospholipid distributions in purple phototrophic bacteria and LH1-RC core complexes[査読有り]
- 2019年03月, SCIENTIFIC REPORTS, 9(1) (1), 3636 - 3636, 英語, 国際誌[査読有り]研究論文(学術雑誌)
- 2018年08月, CHEMISTRY LETTERS, 47(8) (8), 1071 - 1074, 英語[査読有り]研究論文(学術雑誌)
- 2018年07月, BIOCHEMISTRY, 57(30) (30), 4496 - 4503, 英語[査読有り]研究論文(学術雑誌)
- 2018年03月, PHOTOSYNTHESIS RESEARCH, 135(1-3) (1-3), 23 - 31, 英語[査読有り]研究論文(学術雑誌)
- 2017年10月, Proc. Nat. Aca. Sci., 114(41) (41), 10906 - 10911, 英語[査読有り]研究論文(学術雑誌)
- 2017年05月, JOURNAL OF PHYSICAL CHEMISTRY B, 121(19) (19), 5025 - 5032, 英語[査読有り]研究論文(学術雑誌)
- 2016年12月, JOURNAL OF PHYSICAL CHEMISTRY B, 120(49) (49), 12466 - 12473, 英語[査読有り]研究論文(学術雑誌)
- 2016年11月, BIOCHEMISTRY, 55(47) (47), 6495 - 6504, 英語[査読有り]研究論文(学術雑誌)
- 2015年04月, PHOTOSYNTHESIS RESEARCH, 124(1) (1), 19 - 29, 英語[査読有り]研究論文(学術雑誌)
- 一般社団法人 日本生物物理学会, 2014年, 生物物理, 54(1) (1), S183, 英語
- 一般社団法人 日本生物物理学会, 2014年, 生物物理, 54(1) (1), S183, 英語
- 2014年01月, FOOD CHEMISTRY, 143, 452 - 458, 英語[査読有り]研究論文(学術雑誌)
- 2013年12月, BIOCHEMISTRY, 52(50) (50), 9001 - 9008, 英語[査読有り]研究論文(学術雑誌)
- 2013年12月, PHOTOSYNTHESIS RESEARCH, 118(3) (3), 249 - 258, 英語[査読有り]研究論文(学術雑誌)
- 2013年, Photosynthesis Research for Food, Fuel and the Future, Advanced Topics in Science and Technology in China, 105 - 109, 英語Strontium ions are functionally replaceable with calcium ions in the light-harvesting 1 reaction center core complex from Thermophilic purple sulfur bacterium Thermochromatium tepidum[査読有り]研究論文(国際会議プロシーディングス)
- 2012年08月, PLANTA, 236(2) (2), 753 - 761, 英語[査読有り]研究論文(学術雑誌)
- 2012年08月, BIOCHEMISTRY, 51(33) (33), 6556 - 6567, 英語[査読有り]研究論文(学術雑誌)
- 2012年07月, Biochimica et Biophysica Acta, 1817(7) (7), 1022 - 1029, 英語[査読有り]研究論文(学術雑誌)
- 一般社団法人 日本生物物理学会, 2012年, 生物物理, 52, S181, 英語
- 一般社団法人 日本生物物理学会, 2012年, 生物物理, 52, S180 - S181, 英語
- Springer Science and Business Media LLC, 2011年08月, Nature Precedings
Abstract Plants are often exposed to temperatures of around 40^o^C. These temperatures can cause serious damage to photosystems, yet plants can survive with minimum damage. Here, we show that plants switch photosystem to protect photosystem II (PSII) at 40^o^C. Using wheat and Arabidopsis seedlings, we investigated the mechanisms of heat-derived damage in the dark and avoidance of damage in the light. Heat treatment at 40^o^C in the dark caused serious damage to PSII: the maximum quantum yield of PSII (Fv/Fm) and oxygen evolution rapidly decreased. The damage was due to the degradation of the D1 protein (shown by immuno-chemical analysis) and the disturbance of energy transfer in PSII core chlorophyll-binding proteins CP43 and CP47 (shown by time-resolved fluorescence measurement). The damage to PSII might be due to enhanced introduction of electrons from the reducing power of the stroma into thylakoid membranes, causing subsequent electron backflow to PSII. Plants treated at 40^o^C in the light avoided PSII damage and showed preferential excitation of photosystem I (PSI), phosphorylation and migration of light-harvesting complex II (LHCII), which indicate state transition of the photosystem to enhance thermal dispersion and light-driven cyclic electron flow around PSI. These results suggest that heat damage to PSII is probably due to a backflow of reducing power from the stroma to PSII, and that light causes a state transition of photosystem, driving cyclic electron flow and thus protecting PSII from damage.研究論文(学術雑誌) - 2011年05月, BIOCHEMISTRY, 50(18) (18), 3638 - 3648, 英語[査読有り]研究論文(学術雑誌)
- 一般社団法人 日本生物物理学会, 2010年, 生物物理, 50(2) (2), S133, 英語
- 2009年10月, PHOTOSYNTHESIS RESEARCH, 102(1) (1), 77 - 84, 英語[査読有り]研究論文(学術雑誌)
- 2009年03月, FEBS JOURNAL, 276(6) (6), 1739 - 1749, 英語[査読有り]研究論文(学術雑誌)
- 2009年, Optics InfoBase Conference Papers, 英語On the excitation-trap dynamics, red absorption and thermal stability of the lh1-rc complex from photosynthetic bacterium thermochromatium tepidum研究論文(国際会議プロシーディングス)
- 2009年01月, JOURNAL OF BIOLOGICAL CHEMISTRY, 284(1) (1), 93 - 99, 英語[査読有り]研究論文(学術雑誌)
- 2008年10月, BIOPHYSICAL JOURNAL, 95(7) (7), 3349 - 3357, 英語[査読有り]研究論文(学術雑誌)
- 2008年05月, JOURNAL OF BIOLOGICAL CHEMISTRY, 283(20) (20), 13867 - 13873, 英語[査読有り]研究論文(学術雑誌)
- 2007年08月, BIOCHIMICA ET BIOPHYSICA ACTA-BIOENERGETICS, 1767(8) (8), 1057 - 1063, 英語[査読有り]研究論文(学術雑誌)
- 2006年03月, PLANT AND CELL PHYSIOLOGY, 47(3) (3), 419 - 425, 英語[査読有り]研究論文(学術雑誌)
- 2005年12月, BIOCHEMISTRY, 44(49) (49), 16072 - 16078, 英語[査読有り]研究論文(学術雑誌)
- 2005年09月, 280(45) (45), 37895 - 37900Changes in Structural and Functional Properties of Oxygen-evolving Complex Induced by Replacement of D1-Glutamate 189 with Glutamine in Photosystem II LIGATION OF GLUTAMATE 189 CARBOXYLATE TO THE MANGANESE CLUSTER*[査読有り]
- 2005年06月, PHOTOSYNTHESIS RESEARCH, 84(1-3) (1-3), 245 - 250, 英語[査読有り]研究論文(学術雑誌)
- 2005年05月, BIOCHEMISTRY, 44(21) (21), 7613 - 7622, 英語[査読有り]研究論文(学術雑誌)
- 2005年01月, JOURNAL OF BIOLOGICAL CHEMISTRY, 280(3) (3), 2078 - 2083, 英語[査読有り]研究論文(学術雑誌)
- 2004年11月, Biochemistry, 43(46) (46), 14644 - 14652, 英語[査読有り]研究論文(学術雑誌)
- 2004年07月, Journal of Biological Chemistry, 279(28) (28), 29622 - 29627, 英語[査読有り]研究論文(学術雑誌)
- 2004年06月, BIOCHEMISTRY, 43(23) (23), 7479 - 7490, 英語[査読有り]研究論文(学術雑誌)
- 2004年02月, BIOPHYSICAL JOURNAL, 86(2) (2), 1042 - 1050, 英語Oxidation of the Mn cluster induces structural changes of NO3- functionally bound to the Cl- site in the oxygen-evolving complex of photosystem II[査読有り]研究論文(学術雑誌)
- 2003年11月, BIOCHEMISTRY, 42(45) (45), 13170 - 13177, 英語[査読有り]研究論文(学術雑誌)
- 2003年10月, JOURNAL OF INORGANIC BIOCHEMISTRY, 97(2) (2), 231 - 239, 英語[査読有り]研究論文(学術雑誌)
- 一般社団法人 日本生物物理学会, 2003年, 生物物理, 43, S202, 日本語
- 一般社団法人 日本生物物理学会, 2003年, 生物物理, 43, S203, 日本語
- 一般社団法人 日本生物物理学会, 2003年, 生物物理, 43, S202, 日本語
- 2002年11月, BIOCHEMISTRY, 41(46) (46), 13839 - 13850, 英語[査読有り]研究論文(学術雑誌)
- 2002年05月, BIOCHEMISTRY, 41(18) (18), 5844 - 5853, 英語[査読有り]研究論文(学術雑誌)
- 一般社団法人 日本生物物理学会, 2002年, 生物物理, 42(2) (2), S108, 日本語
- 一般社団法人 日本生物物理学会, 2002年, 生物物理, 42(2) (2), S108, 日本語
- 2001年11月, BIOCHEMISTRY, 40(46) (46), 14061 - 14068, 英語Chelator-induced disappearance of carboxylate stretching vibrational modes in S-2/S-1 FTIR spectrum in oxygen-evolving complex of photosystem II[査読有り]研究論文(学術雑誌)
- Royal Society of Chemistry (RSC), 1998年, Journal of the Chemical Society, Faraday Transactions, 94(20) (20), 3077 - 3085[査読有り]研究論文(学術雑誌)
- American Chemical Society (ACS), 1997年01月, The Journal of Physical Chemistry A, 101(4) (4), 459 - 465[査読有り]研究論文(学術雑誌)
- 2023年11月, The 61th Annual Meeting of the BSJPurification and characterization of a new thermophilic purple sulfur bacterium Caldichromatium japonicum
- 2023年06月, 第29回光合成セミナー:反応中心と色素系の多様性(名古屋), 日本語新規好熱性紅色光合成細菌Caldichromatium japonicmの特性解析
- 2023年06月, 第29回光合成セミナー:反応中心と色素系の多様性(名古屋), 日本語紅色光合成細菌由来カルシウム結合型LH1-RC複合体の構造機能解析
- 2022年09月, The 60th Annual Meeting of the BSJ, Hakodate,, 英語FTIR monitoring of photosynthetic quinone transport in the light-harvesting 1 reaction center complex from purple bacteria
- 2022年09月, The 60th Annual Meeting of the BSJ, Hakodate, 英語Electrostatic charge controls spectral properties and thermal stabilities of LH1-RCs from triply extremophilic Halorhodospira halochloris
- 2022年03月, 日本化学会第102春季年会(兵庫), 日本語紅色光合成細菌Thermochromatium tepidumのLH2タンパク質のB800バクテリオクロロフィルaの酸化による分光特性変化
- 2021年09月, 第15回バイオ関連化学シンポジウム、鳥取紅色光合成細菌Thermochromatium tepidumのLH2タンパク質に結合するB800バクテリオクロロフィルaの選択的酸化
- 2021年09月, 第15回バイオ関連化学シンポジウム、鳥取, 日本語紅色光合成細菌の光捕集タンパク質LH2へ再構成したホルミル基含有クロロフィルのスペクトル特性
- 2021年07月, 第28回光合成セミナー:反応中心と色素系の多様性、豊中, 日本語Thermochromatium tepidumのLH2タンパク質のB800の結合状態と全体構造の解析
- 2021年07月, 第28回光合成セミナー:反応中心と色素系の多様性、豊中, 日本語LH2タンパク質のB800部位へのクロロフィルb/d/fの再構成:分光特性とタンパク質との相互作用
- 2021年07月, 第28回光合成セミナー:反応中心と色素系の多様性、豊中, 日本語カロテノイド生合成系の調節による紅色細菌の熱耐性への適応
- 2021年07月, 第28回光合成セミナー:反応中心と色素系の多様性、豊中, 日本語3つの好極限性を有する紅色光合成細菌Halorhodospira halochlorisの特性解析
- 2021年07月, 第28回光合成セミナー:反応中心と色素系の多様性、豊中, 日本語紅色光合成細菌のキノン輸送におけるリン脂質の寄与
- 2021年06月, 第47回生体分子科学討論会、兵庫, 日本語紅色光合成細菌のLH12タンパク質へのホルミル基を有するクロロフィルの再構成”
- 2019年09月, 日本農芸化学会 関西・中部支部2019合同神戸大会(神戸)好熱性紅色光合成細菌Halorhodospira halochlorisの特性解析
- 2019年09月, 日本農芸化学会 関西・中部支部2019合同神戸大会(神戸)紅色光合成細菌電子伝達系におけるキノン輸送の追跡
- 2019年07月, 第27回「光合成セミナー2019:反応中心と色素系の多様性」(豊中)紅色光合成細菌におけるキノン動態の解析
- 2018年07月, 第26回「光合成セミナー2018:反応中心と色素系の多様性」(神戸)好熱性および好塩性を示す Halorhodospira halochloris由来光捕集反応中心複合体の特性解析
- 2018年07月, 第26回「光合成セミナー2018:反応中心と色素系の多様性」(神戸)光誘起FTIR分光法を用いた紅色細菌由来LH1-RC複合体におけるキノン分子の追跡
- 2018年07月, 第26回「光合成セミナー2018:反応中心と色素系の多様性」(神戸)紅色光合成細菌 Thiorhodovibrio Strain 970が示す異常なLH1Qyレッドシフトの起源
- 2017年09月, 第55回日本生物物理学会年会(熊本)Monitoring of quinone reduction in the thermophilic purple bacterium Thermochromatium tedium by means of isotope-edited FTIR spectroscopy
- 2017年07月, 第25回「光合成セミナー2017:反応中心と色素系の多様性」(神戸)同位体標識化された好熱性紅色細菌Thermochromatium tediumにおけるキノール分子の振動分光学的検出
- 2017年03月, 第58回日本植物生理学会年会FTIR 分光法を用いた好熱性紅色細菌 Thermochromatium tepidumにおける光合成電子伝達反応のモニタリング
- 2016年11月, 第54回日本生物物理学会年会(東京)好熱性紅色光合成細菌Thermochromatium tepidum 由来反応中心複合体におけるカルシウムイオンの機能的、構造的役割
- 2006年, PLANT AND CELL PHYSIOLOGY, 47, S28 - S28, 英語Ligation structure of the Mn cluster in photosynthetic oxygen evolving complex studied by FTIR spectroscopy研究発表ペーパー・要旨(国際会議)
- 2006年, PLANT AND CELL PHYSIOLOGY, 47, S27 - S27, 英語Property changes of the photosynthetic oxygen-evolving complex induced by replacement of D1-Glu189 with Gln研究発表ペーパー・要旨(国際会議)
- 2005年, PLANT AND CELL PHYSIOLOGY, 46, S26 - S26, 英語Carboxylate of D1 C-terminal Ala344 ligates a Mn ion that is oxidized upon S-1-to-S-2 transition and reduced upon S-3-to-S-0 transition研究発表ペーパー・要旨(国際会議)
- 2005年, PLANT AND CELL PHYSIOLOGY, 46, S179 - S179, 英語Effects of systematic replacement of D1 C-terminal Ala344 with Asn, Asp, Gln, or Glu on the properties of photosynthetic oxygen evolving complex研究発表ペーパー・要旨(国際会議)
- 2005年, PLANT AND CELL PHYSIOLOGY, 46, S26 - S26, 英語FTIR detection of water-sensitive low-frequency vibrational modes during photosynthetic water oxidation in photosystem II研究発表ペーパー・要旨(国際会議)
- 2005年, Photosynthesis: Fundamental Aspects to Global Perspectives, 376 - 378Functional implications of the D1 C-terminal α-carboxylate in photosynthetic oxygen evolution as studied by FTIR
- 2005年, Photosynthesis: Fundamental Aspects to Global Perspectives, 352 - 354Functional and structural properties of oxygen-evolving complex in D1 C-terminal mutants
- 2004年, PLANT AND CELL PHYSIOLOGY, 45, S83 - S83, 英語Low-frequency FTIR difference spectra for S-state cycling of photosynthetic water oxidation研究発表ペーパー・要旨(国際会議)
- 2004年, PLANT AND CELL PHYSIOLOGY, 45, S81 - S81, 英語Characteristics of oxygen-evolving system in Synechocystis mutants on C-terminus of D1 protein of photosystem II: (2) An FTIR study研究発表ペーパー・要旨(国際会議)
- 2002年, PLANT AND CELL PHYSIOLOGY, 43, S64 - S64, 英語Characteristic change in the Ligation structure of the Mn-cluster in photosystem II induced by Ca2+-depletion and metal chelators addition as revealed by FTIR spectroscopy研究発表ペーパー・要旨(国際会議)
- 共著, 光アライアンス・日本工業出版, 2024年09月紅色硫黄細菌の光合成とCa2+ カルシウムイオン欠乏環境での光合成戦略
- 共著, InTech, 2012年, 英語Function of Extrinsic Proteins in Stabilization of the Photosynthetic Oxygen-Evolving Complex学術書
- 共著, Nova Science Publishers, 2011年, 英語Photosystem at high temperature -mechanisms of adaptation and damage学術書
- AOICP2024, 2024年09月, 英語The LH1–RC structure of a thermophilic purple nonsulfur bacterium powers photosynthesis with extremely low-energy near-infrared lightポスター発表
- AOICP2024, 2024年09月, 英語Characterization of the light-harvesting 1 reaction center complex from psychrophilic purple nonsulfur bacteriaポスター発表
- AOICP2024, 2024年09月, 英語The most stable LH1-RC structure from a novel thermophilic purple sulfur bacterium, Caldichromatium japonicumポスター発表
- 21st IUPAB & 62nd BSJ JOINT CONGRESS 2024, 2024年06月, 英語Structural Basis for Enabling Photosynthesis with Extremely Low-energy Near-infrared Light in the LH1–RC Complex of a Thermophilic Purple Nonsulfur Bacteriumポスター発表
- 21st IUPAB & 62nd BSJ JOINT CONGRESS 2024, 2024年06月, 英語Characterization of an LH1–RC photocomplex from a novel Japanese hot spring purple sulfur bacterium, Caldichromatium japonicumポスター発表
- 第31回 光合成セミナー2024:反応中心と色素系の多様性, 2024年06月, 日本語好冷性紅色光合成細菌における光捕集1反応中心複合体の特性解析ポスター発表
- 第31回 光合成セミナー2024:反応中心と色素系の多様性, 2024年06月, 日本語好熱性紅色硫黄細菌Caldichromatium japonicumにおけるLH1-RC耐熱化の分子機構口頭発表(一般)
- 第31回 光合成セミナー2024:反応中心と色素系の多様性, 2024年06月, 日本語B800バクテリオクロロフィルaの脱離がLH2タンパク質の構造・機能に与える影響口頭発表(一般)
- 第31回 光合成セミナー2024:反応中心と色素系の多様性, 2024年06月, 日本語紅色非硫黄細菌Blastochloris tepida におけるLH1-RC複合体の構造機能解析ポスター発表
- The 60th Annual Meeting of the BSJ, 2023年11月, 英語Purification and characterization of a new thermophilic purple sulfur bacterium Caldichromatium japonicumポスター発表
- 第23回日本光生物学協会年会, 2023年08月, 日本語紅色光合成細菌のLH2タンパク質のB800バクテリオクロロフィルaの脱離:タンパク質への影響の解析ポスター発表
- 第30回 光合成セミナー 2023年6月, 2023年06月, 日本語紅色光合成細菌由来カルシウム結合型LH1-RC複合体の構造機能解析[招待有り]口頭発表(招待・特別)
- 第30回 光合成セミナー 2023年6月, 2023年06月, 日本語新規好熱性紅色光合成細菌Caldichromatium japonicumの特性解析ポスター発表
- 第45回 日本分子生物学会年会Cryo-EM structure of a LH1–RC Photocomplex from an Extremophilic Phototroph
- The 60th Annual Meeting of the BSJ, 2022年09月FTIR monitoring of photosynthetic quinone transport in the light-harvesting 1 reaction center complexes from purple bacteriaポスター発表
- The 60th Annual Meeting of the BSJ, 2022年09月Electrostatic charge controls spectral properties and thermal stabilities of LH1-RCs from triply extremophilic Halorhodospira halochlorisポスター発表
- 第16回バイオ関連化学シンポジウム, 2022年09月紅色光合成細菌Thermochromatium tepidumのLH2タンパク質に結合するバクテリオクロロフィルaの化学酸化と光酸化口頭発表(一般)
- 第29回 光合成セミナー, 2022年07月好極限性紅色細菌Halorhodospira halochlorisにおける低エネルギー吸収特性および3重耐性の分子機構口頭発表(一般)
- 第15回バイオ関連化学シンポジウム, 2021年09月紅色光合成細菌の光捕集タンパク質LH2へ再構成したホルミル基含有クロロフィルのスペクトル特性
- 第15回バイオ関連化学シンポジウム, 2021年09月紅色光合成細菌Thermochromatium tepidumのLH2タンパク質に結合するB800バクテリオクロロフィルaの選択的酸化
- 第28回光合成セミナー:反応中心と色素系の多様性, 2021年07月紅色光合成細菌のキノン輸送におけるリン脂質の寄与
- 第28回光合成セミナー:反応中心と色素系の多様性, 2021年07月3つの好極限性を有する紅色光合成細菌Halorhodospira halochlorisの特性解析
- 第28回光合成セミナー:反応中心と色素系の多様性, 2021年07月カロテノイド生合成系の調節による紅色細菌の熱耐性への適応
- 第28回光合成セミナー:反応中心と色素系の多様性, 2021年07月LH2タンパク質のB800部位へのクロロフィルb/d/fの再構成:分光特性とタンパク質との相互作用
- 第28回光合成セミナー:反応中心と色素系の多様性, 2021年07月Thermochromatium tepidumのLH2タンパク質のB800の結合状態と全体構造の解析
- SNCPP21Oxidation of B800 Bacteriochlorophyll a in LH2 Protein from the Purple Photosynthetic Bacterium Thermochromatium tepidum
- 第47回生体分子科学討論会, 2021年06月紅色光合成細菌のLH12タンパク質へのホルミル基を有するクロロフィルの再構成
- 日本農芸化学会 関西・中部支部2019合同神戸大会, 2019年09月, 日本語, 神戸好熱性紅色光合成細菌Halorhodospira halochlorisの特性解析
- 日本農芸化学会 関西・中部支部2019合同神戸大会, 2019年09月, 日本語, 神戸バクテリオクロロフィルbを有する好熱性紅色細菌Blastochloris tepidaの耐熱性獲得機構
- 日本農芸化学会 関西・中部支部2019合同神戸大会, 2019年09月, 日本語, 神戸紅色光合成細菌電子伝達系におけるキノン輸送の追跡
- 第27回光合成セミナー:反応中心と色素系の多様性, 2019年07月, 日本語, 豊中新規好熱性紅色細菌Blastochloris tepida由来LH1-RCの耐熱化機構と低エネルギー吸収特性の要因
- 第27回光合成セミナー:反応中心と色素系の多様性, 2019年07月, 日本語, 豊中紅色光合成細菌におけるキノン動態の解析”、第27回光合成セミナー:反応中心と色素系の多様性
- 第27回光合成セミナー:反応中心と色素系の多様性, 2019年07月, 日本語, 豊中紅色光合成細菌由来光捕集1反応中心複合体のスペクトル多様性と構造安定性におけるカルシウムイオンの役割
- 日本化学会第99春季年会, 2019年03月, 英語, 甲南大学, 国内会議Product analysis of electrochemical oxidation of polyphenols口頭発表(一般)
- The 57th Annual Meeting of the BSJ, 2019年, 英語, MiyazakiA dual role for calcium in expanding the spectral diversity and stability of LH1-RC photocomplexes of purple phototrophic bacteria
- The 57th Annual Meeting of the BSJ, 2019年, 英語, MiyazakiSpectroscopic characterization of a bacteriochlorophyll b-based LH1-RC complexes from thermophilic purple bacterium Blastochloris tepida
- 若手フロンティア研究会, 2018年12月, 日本語, 神戸大学百年記念館, 国内会議ポリフェノールの電解酸化機構の解明ポスター発表
- 第64回ポーラログラフィーおよび電気分析化学討論会, 2018年11月, 日本語, 壱岐の島ホール(長崎県), 国内会議全電解-HPLCによるポリフェノール電解酸化機構の比較ポスター発表
- 日本分析化学会第68年会, 2018年09月, 日本語, 日本分析化学会, 東北大学, 国内会議ポリフェノールの電解酸化生成物の比較口頭発表(一般)
- The 56th Annual Meeting of the BSJ, 2018年09月, 英語, Okayama, 国内会議Where is the quinone gate in purple photosynthetic bacterial LH1-RC complex?シンポジウム・ワークショップパネル(指名)
- The 56th Annual Meeting of the BSJ, 2018年09月, 英語, Okayama, 国内会議Origin of the anomalous uphill energy gap in the light-harvesting reaction center from purple photosynthetic bacterium strain 970口頭発表(一般)
- The 56th Annual Meeting of the BSJ, 2018年09月, 英語, Okayama, 国内会議Light-induced FTIR spectroscopic studies on quinone exchange mechanism of the LH1-RC complexes from native and chimeric purple bacteriaポスター発表
- 日本分析化学会中部支部・近畿支部合同夏期セミナー, 2018年08月, 日本語, 日本分析化学会, 福井大学, 国内会議ポリフェノールの電解酸化機構の解明ポスター発表
- 第26回光合成セミナー:反応中心と色素系の多様性, 2018年07月, 日本語, 神戸, 国内会議同位体標識化された好熱性紅色硫黄細菌由来LH1-RC複合体の振動分光学的解析ポスター発表
- 第26回光合成セミナー:反応中心と色素系の多様性, 2018年07月, 日本語, 神戸, 国内会議紅色光合成細菌Thiorhodovibrio Strain 970が示す異常なLH1 Qyレッドシフトの起源口頭発表(一般)
- 第26回光合成セミナー:反応中心と色素系の多様性, 2018年07月, 日本語, 神戸, 国内会議好熱性および好塩性を示すHalorhodospira halochloris由来光捕集反応中心複合体の特性解析ポスター発表
- 第26回光合成セミナー:反応中心と色素系の多様性, 2018年07月, 日本語, 神戸, 国内会議光誘起FTIR分光法を用いた紅色細菌由来LH1-RC複合体におけるキノン分子の追跡ポスター発表
- 第26回光合成セミナー:反応中心と色素系の多様性, 2018年07月, 日本語, 神戸, 国内会議LH2タンパク質のB800バクテリオクロロフィルaのクロリン環色素への置換口頭発表(一般)
- 第9回日本光合成学会, 2018年05月, 日本語, 仙台, 国内会議ヘリオバクテリア膜の光駆動キノン還元反応をFTIR法により検出する試みポスター発表
- 第78回分析化学討論会, 2018年05月, 日本語, 日本分析化学会, 山口大学, 国内会議カフェイン酸の電解酸化に伴う再還元能獲得に対する溶媒の影響口頭発表(一般)
- 第9回日本光合成学会, 2018年05月, 日本語, 仙台, 国内会議BChl bをもつ好熱性紅色非硫黄細菌Blastochloris tepida由来LH1-RCの特性評価ポスター発表
- 第9回日本光合成学会, 2018年05月, 日本語, 仙台, 国内会議963 nmにQy遷移をもつ紅色細菌Thiorhodovibrio strain 970由来LH1-RCの特性評価ポスター発表
- 第63回ポーラログラフィーおよび電気分析化学討論会, 2017年11月, 日本語, 海峡メッセ下関, 国内会議カフェイン酸の電解酸化機構の解析口頭発表(一般)
- 日本分析化学会第66年会, 2017年09月, 日本語, 東京理科大学, 国内会議カフェイン酸の電解酸化機構の解明口頭発表(一般)
- The 55nd Annual Meeting of the BSJ, 2017年09月, 英語, Kumamoto, 国内会議Monitoring of quinone reduction in the thermophilic purple bacterium Thermochromatium tedium by means of isotope-edited FTIR spectroscopy口頭発表(一般)
- The 55th Annual Meeting of the BSJ, 2017年09月, 英語, Kumamoto, 国内会議Co-crystallization of a bacterial photosynthetic electron-transfer complexポスター発表
- 第25回光合成セミナー:反応中心と色素系の多様性, 2017年07月, 日本語, 神戸, 国内会議同位体標識化された好熱性紅色細菌Thermochromatium tediumにおけるキノール分子の振動分光学的検出ポスター発表
- 第25回光合成セミナー:反応中心と色素系の多様性, 2017年07月, 日本語, 神戸, 国内会議新規好熱性紅色細菌Allochromatium tepidum由来LH1-RC複合体におけるCa依存型耐熱化機構のATR-FTIR分析ポスター発表
- 第25回光合成セミナー:反応中心と色素系の多様性, 2017年07月, 日本語, 神戸, 国内会議Thermochromatium tepidumおよびRhodobacter sphaeroides由来LH1-RCキメラ複合体の特性解析口頭発表(一般)
- the 5th Awaji International Workshop on Electron Spin Science & Technology, 2017年06月, 英語, Awaji, Hyogo, 国際会議Spectroscopic and thermodynamic analyses of the LH1-RC complexes from a new thermophilic purple bacterium Allochromatium tepidu[招待有り]口頭発表(招待・特別)
- 8th OCARINA International Symposium, 2017年03月, 英語, Osaka, 国際会議Spectroscopic and thermodynamic characterization of LH1-RC complexes from Thermophilic purple bacteria[招待有り]口頭発表(招待・特別)
- 第58回日本植物生理学会年会, 2017年03月, 日本語, 鹿児島, 国内会議FTIR 分光法を用いた好熱性紅色細菌 Thermochromatium tepidumにおける光合成電子伝達反応のモニタリング口頭発表(一般)
- 日本農芸化学会関西支部第497回講演会, 2016年12月, 日本語, 神戸, 国内会議新規好熱性紅色光合成細菌Allochromatium tepidum由来LH1-RCの耐熱性制御機構口頭発表(一般)
- 若手フロンティア研究会2016, 2016年12月, 日本語, 神戸, 国内会議異種複合体発現系を用いたuphill型光合成反応の分子機構解析ポスター発表
- 日本農芸化学会関西支部第497回講演会, 2016年12月, 日本語, 神戸, 国内会議ヘテロ複合体発現系を用いたuphill型光合成反応機構の解析口頭発表(一般)
- The 54nd Annual Meeting of the BSJ, 2016年11月, 英語, Tsukuba, 国内会議Spectroscopic characterization of site-directed mutants in light-harvesting 1 complex from Thermochromatium tepidumポスター発表
- The 54nd Annual Meeting of the BSJ, 2016年11月, 英語, Tsukuba, 国内会議Purification and spectroscopic characterization of the light-harvesting complexes from thermophilic purple bacterium Allochromatium tepidumポスター発表
- The 54nd Annual Meeting of the BSJ, 2016年11月, 英語, Tsukuba, 国内会議Functional and structural roles of calcium ion in the reaction center complex from thermophilic purple photosynthetic bacteriumポスター発表
- 17th International Congress on Photosynthesis, 2016年08月, 英語, Maastricht, The Netherlands, 国際会議Spectroscopic and thermodynamic characterization of the metal-binding sites in the LH1-RC complex from thermophilic purple sulfur bacterium Thermochromatium tepidumポスター発表
- 17th International Congress on Photosynthesis, 2016年08月, 英語, Maastricht, The Netherlands, 国際会議Crystal structures reveal molecular basis for the bacterial LH1 Qy transition and its thermostabilityポスター発表
- Light-harvesting satellite meeting of 17th International Congress on Photosynthesis, 2016年08月, 英語, Egmond aan Zee, The Netherlands, 国際会議Characterization of the LH complexes from a new thermophilic purple bacterium Allochromatium tepidumポスター発表
- 17th International Congress on Photosynthesis, 2016年08月, 英語, Maastricht, The Netherlands, 国際会議Characterization of the LH complexes from a new thermophilic purple bacterium Allochromatium tepidumポスター発表
- 第24回光合成セミナー:反応中心と色素系の多様性, 2016年07月, 日本語, 京都, 国内会議新規好熱性紅色硫黄細菌Allochromatium tepidum由来LH1-RCの特性解析ポスター発表
- 第24回光合成セミナー:反応中心と色素系の多様性, 2016年07月, 日本語, 京都, 国内会議888nmにQy遷移をもつ紅色細菌LH1の構造解析口頭発表(一般)
- The 4th Awaji International Workshop on Electron Spin Science & Technology, 2016年06月, 英語, Awaji, Hyogo, 国際会議Effects of metal cations on charge separated states of the reaction center from thermophilic purple photosynthetic bacterium Thermochromatium tepidumポスター発表
- 植物生理学会年会 第一回光合成細菌ワークショップ, 2016年03月, 日本語, 盛岡, 国内会議好熱性紅色細菌Thermochromatium tepidum由来光捕集1反応中心複合体における 吸収特性および耐熱性制御の分子機構[招待有り]口頭発表(招待・特別)
- The 96th Annual Meeting 2016 of CSJ, 2016年03月, 英語, Kyoto, 国内会議Analysis of Molecular Mechanism for the Enhanced Stability of Flavonoid Compoundsポスター発表
- The International Chemical Congress of Pacific Basin Societies, Honolulu, 2015年12月, 英語, 国際会議Molecular mechanisms for enhanced thermal and light stabilities of flavonoids by metal cations and polysaccharidesポスター発表
- The 53nd Annual Meeting of the BSJ, 2015年09月, 英語, Kanazawa, 国内会議Towards elucidating the unusual absorption behavior and enhanced thermostability of the LH1-RC complex from Thermochromatium tepidumポスター発表
- The 53nd Annual Meeting of the BSJ, 2015年09月, 英語, Kanazawa, 国内会議Monitoring of metal-binding sites and metal-protein interactions in the LH1-RC complex from thermophilic purple photosynthetic bacteriaポスター発表
- The 53nd Annual Meeting of the BSJ, 2015年09月, 英語, Kanazawa, 国内会議Molecular mechanisms for the enhanced thermal stability of LH1-RC complex from Thermochromatium tepidum: isotope-edited FTIR spectroscopyポスター発表
- The 53nd Annual Meeting of the BSJ, 2015年09月, 英語, Kanazawa, 国内会議Detection of quinone molecules in the LH1-RC complex from the thermophilic purple photosynthetic bacterium Thermochromatium tepidumポスター発表
- 第23回光合成セミナー:反応中心と色素系の多様性, 2015年07月, 日本語, 京都, 国内会議好熱性紅色細菌Thermochromatium tepidum由来光捕集1反応中心複合体におけるCa2+結合サイトの特性解析口頭発表(一般)
- The 52nd Annual Meeting of the BSJ, 2014年09月, 英語, Sapporo, Hokkaido, 国内会議Thermodynamic analysis of metal-protein interaction in the light-harvesting 1 reaction center complex from purple sulfur bacteriaポスター発表
- The 52nd Annual Meeting of the BSJ, 2014年09月, 英語, Sapporo, Hokkaido, 国内会議Isotope-edited ATR-FTIR analysis of light-harvesting 1 reaction center complex from thermophilic purple photosynthetic bacteriaポスター発表
- 第22回光合成セミナー:反応中心と色素系の多様性, 2014年07月, 日本語, 名古屋, 国内会議同位体置換された好熱性紅色細菌Thermochromatium tepidum由来光捕集1反応中心複合体の振動分光学的解析ポスター発表
- 第22回光合成セミナー:反応中心と色素系の多様性, 2014年07月, 日本語, 名古屋, 国内会議好熱性紅色細菌由来光捕集1反応中心複合体のメタロミクス解析ポスター発表
- The 2nd Awaji International Workshop on Electron Spin Science & Technology, 2014年06月, 英語, Awaji, Hyogo, 国際会議Metal cations modulate protein conformation and thermal stability of light-harvesting 1 reaction center complexes from purple sulfur photosynthetic bacteria[招待有り]口頭発表(招待・特別)
- 日本化学会第93春季年会, 2013年03月, 日本語, 国内会議鉄(III)及び酸性多糖類共存系におけるアントシアニンの耐久性及び機能評価口頭発表(一般)
- 日本化学会第93春季年会, 2013年03月, 日本語, 国内会議全反射吸収赤外分光法による紅色硫黄光合成細菌Thermochromatium tepidum由来光捕集1膜蛋白質複合体の構造解析”、 日本化学会第93春季年会口頭発表(一般)
- 第20回光合成の色素系と反応中心に関するセミナー, 2013年, 日本語, 国内会議好熱性紅色細菌由来光捕集1反応中心複合体の耐熱化を制御する構造変化の振動分光学的検出口頭発表(一般)
- 16th International Congress on Photosynthesis, 2013年, 英語, 国際会議Molecular mechanisms for the enhanced thermal stability and unusually red-shifted Qy transition of light-harvesting 1 complexes from thermophilic purple sulfur bacterium Thermochromatium tepidum as revealed by vibrational spectroscopyポスター発表
- 第473回日本農芸化関西支部講演会, 2012年, 日本語, 国内会議灌流誘起赤外分光法による光合成膜蛋白質複合体の構造解析口頭発表(一般)
- 第53回日本植物生理学会年会, 2012年, 日本語, 国内会議高温ストレスを受けた植物がステート遷移によりPSIIを保護するメカニズムの解析口頭発表(一般)
- 第20回光合成の色素系と反応中心に関するセミナー, 2012年, 日本語, 国内会議紅色硫黄細菌由来光捕集1複合体における色素—蛋白質相互作用の解析口頭発表(一般)
- 日本化学会第92春季年会, 2012年, 日本語, 国内会議紅色硫黄光合成細菌由来cytochrome c’の熱安定性におけるC末端近傍アミノ酸残基の影響ポスター発表
- 第50回日本生物物理学会年会, 2012年, 英語, 国内会議好熱性紅色硫黄細菌Thermochromatium tepidum由来光捕集1複合体におけるBChl-aとTrp残基間の水素結合相互作用ポスター発表
- 第50回日本生物物理学会年会, 2012年, 英語, 国内会議好熱性紅色硫黄細菌Thermochromatium tepidum由来cytochrome c’における耐熱化メカニズムの検討ポスター発表
- 日本化学会第92春季年会, 2012年, 日本語, 国内会議金属及び機能性多糖類によるアントシアニンの耐熱性向上メカニズムの検証ポスター発表
- 日本化学会第92春季年会, 2012年, 日本語, 国内会議金属イオンに制御される好熱性紅色光合成細菌由来光捕集反応中心複合体1の色素―蛋白質間相互作用口頭発表(一般)
- Japan Analytical & Scientific Instruments Show, 2012年, 英語, 国際会議Detection of pigment-protein interactions in photosynthetic purple bacteria by Near Infrared Raman Spectroscopyポスター発表
- 日本農芸化学会関西・中部支部合同大会, 2011年, 日本語, 国内会議植物が光化学系の高温障害を回避するメカニズムの解析口頭発表(一般)
- 第472回日本農芸化関西支部講演会, 2011年, 日本語, 国内会議好熱性紅色細菌Thermochromatium tepidum由来光捕集反応中心複合体の色素—蛋白質間相互作用における金属イオンの役割口頭発表(一般)
- 第38回生体分子科学討論会, 2011年, 日本語, 国内会議好熱性光合成細菌由来の電子伝達タンパク質の機能評価と構造解析口頭発表(一般)
- 第19回光合成の色素系と反応中心に関するセミナー, 2011年, 日本語, 国内会議Thermochromatium tepidum由来光捕集反応中心複合体における金属結合サイトの特性解析口頭発表(一般)
- 第48回日本生物物理学会年会, 2010年, 英語, 国内会議生合成的にSr2+置換された好熱性紅色硫黄細菌Thermochromatium tepidumにおける色素蛋白質複合体の特性評価ポスター発表
- 日本農芸化学会関西支部第467回講演会, 2010年, 日本語, 国内会議光化学系Ⅱ酸素発生複合体における表在性蛋白質PsbPとカルシウムの構造的、機能的役割口頭発表(一般)
- The International Chemical Congress of Pacific Basin Societies, Honolulu, 2010年, 英語, 国際会議Structural and functional roles of metal cations in the Photosynthetic core complex from thermophilic purple sulfur bacterium Thermochromatium tepidumポスター発表
- 15th International Congress on Photosynthesis, 2010年, 英語, 国際会議Strontium Ions Are Functionally Replaceable with Calcium Ions in the Light-Harvesting 1 Reaction Center Core Complex from Thermophilic Purple Sulfur bacterium Thermochromatium tepidumポスター発表
- The International Chemical Congress of Pacific Basin Societies, Honolulu, 2010年, 英語, 国際会議Interaction between dietary fibers and toxic heavy metals as revealed by vibrational specroscopyポスター発表
- 15th International Congress on Photosynthesis, 2010年, 英語, 国際会議Calcium Ions Are Involved in the Thermostability and the Unusual Red-Shift of LH1 Qy Transition of the Core Complex from Thermophilic Purple Sulfur Bacterium Thermochromatium tepidumポスター発表
- 特定領域研究「生体超分子構造」第6回公開シンポジウム, 2009年, 日本語, 国内会議好熱性紅色光合成細菌由来Cytochrome c’の耐熱性に関する研究ポスター発表
- 第9回日本蛋白質科学会年会, 2009年, 日本語, 国内会議好熱性光合成細菌由来のFlavocytochrome cとCytochrome c’の結晶構造解析と機能評価ポスター発表
- 特定領域研究「生体超分子構造」第6回公開シンポジウム, 2009年, 日本語, 国内会議好熱性光合成細菌Thermochromatium tepudum由来光捕集タンパク質—反応中心複合体の構造解析ポスター発表
- 特定領域研究「生体超分子構造」第6回公開シンポジウム, 2009年, 日本語, 国内会議光合成光捕集反応中心複合体におけるCa2+結合部位の検証ポスター発表
- Conference on Lasers and Electro-Optics/Pacific Rim, 2009年, 英語, 国際会議On the Excitation-Trap Dynamics, Red Absorption and Thermal Stability of the LH1-RC Complex from Photosynthetic Bacterium Thermochromatium tepidumポスター発表
■ 共同研究・競争的資金等の研究課題
- 日本学術振興会, 科学研究費助成事業, 学術変革領域研究(A), 神戸大学, 2024年04月01日 - 2026年03月31日紅色硫黄光合成細菌におけるカルシウムを利用した環境適応戦略の解明
- 日本学術振興会, 科学研究費助成事業, 基盤研究(C), 神戸大学, 2022年04月 - 2025年03月極限環境光合成微生物における3重耐性化と近赤外光利用の分子戦略
- 日本学術振興会, 科学研究費助成事業 新学術領域研究(研究領域提案型), 新学術領域研究(研究領域提案型), 神戸大学, 2020年11月 - 2022年03月近赤外光応答型光合成エネルギー変換を担うキノン-キノール輸送機構の解明本研究では紅色細菌由来のLH1-RC、キノン、脂質を用いて光合成膜を再構成する手法を確立し、様々な組み合わせで再構成した膜について、キノン還元からキノール輸送に至るキノン動態をモニタリングすることにより、膜内部におけるキノン動態の直接的な実験的証拠を得ること、ならびにキノン-タンパク質間相互作用やキノン-脂質間相互作用を検証することにより、キノン-キノール輸送経路およびその分子機構を明らかにすることを目的とした。具体的には、(1) 天然および人工再構成膜調製法の確立、(2) 光合成膜およびLH1-RCにおけるキノール生成の検出、(3) 天然再構成膜、人工再構成膜におけるキノール生成の検出、(4) 再構成膜系キノール生成における同位体置換、キノン置換、脂質置換の効果、(5) ATR-FTIRおよびHPLCを用いた遊離キノールの検出、(6) 形状の異なるLH1-RCを用いた比較研究について検討してきた。 本年度は(3)、(4)の人工再構成膜の系について重点的に研究を進めた。主要構成脂質であるCardiolipin、Phosphatidylglycerol、Phosphatidylethanolamine、Phosphatidylcholineを用いた人工膜の再構成条件を最適化し、光誘起赤外分光法によるキノン・キノール由来の信号検出に成功した。(5)については遊離キノンが検出されなかったことから、LH1-RCのキノン輸送において膜脂質の重要性が示唆された。(6)については、C字型のLH1、キノン輸送関連タンパク質であるPuf XやProtein Uを有するRba. sphaeroidesを用いて測定を行なった結果、キノンの還元に対するC字型リング形状、Puf XやProtein U欠損の効果はほとんど見られなかったが、キノンの拡散輸送に対する効果について現在も検証を進めている。
- 科学研究費補助金/基盤研究(C), 2019年04月 - 2022年03月, 研究代表者紅色光合成細菌による近赤外光電変換メカニズムの解明競争的資金
- 学術研究助成基金助成金/基盤研究(C), 2016年04月 - 2019年03月, 研究代表者競争的資金
- 科学研究費補助金/基盤研究(C), 2012年04月 - 2015年03月, 研究代表者競争的資金
- 日本学術振興会, 科学研究費補助金/基盤研究(C), 基盤研究(C), 茨城大学, 2011年 - 2013年金属イオンによって誘起される光合成光捕集複合体の構造と機能変化自然界最古のアンテナ・光電変換機能をもつ光合成分子機械の立体構造を原子レベルの分解能で決定した。その構造情報から光捕集と電荷分離の作動原理およびこれらの機能を制御する金属イオンの役割を明らかにした。また、光捕集を司るアンテナ複合体において色素分子間の幾何学的配置及び色素タンパク質間の相互作用も解明した。これにより、光捕集複合体から反応中心複合体への励起エネルギー移動ならびに反応中心からキノンプールへの電子伝達を規定する構造因子を突き止めた。本研究より得られた成果は、今年のNature誌にArticleとして掲載され,当分野今後の研究の飛躍につながるものと期待される。競争的資金
- 日本学術振興会, 科学研究費補助金/若手研究(B), 若手研究(B), 神戸大学, 2008年 - 2010年, 研究代表者Ca^<2+>との相互作用により耐熱性と異常な吸収特性を示す好熱性紅色細菌Thermochromatium tepidum由来光捕集反応中心複合体の分光学的、熱力学的解析を行い、光捕集複合体C末端近傍とCa^<2+>の相互作用により顕著な構造変化は起こさず、色素の配向状態や蛋白質の熱安定性が精密に制御されていることを明らかにした。また、Sr^<2+>が生合成的にCa^<2+>と置換し得ることを見出し、その特性評価を行った。競争的資金
- 日本学術振興会, 科学研究費補助金/若手研究(B), 若手研究(B), 独立行政法人理化学研究所, 2002年 - 2003年, 研究代表者光合成酸素発生反応機構の解明:マンガンクラスターによる水分解反応の直接検出光化学系IIマンガン(Mn)クラスターの配位構造及びMnクラスターが水を酸化して酸素を発生する反応機構を明らかにすることを目的とした。本年度は下記1-3について研究を行った。 1.低波数(650-350cm^<-1>)赤外分光法により同位体標識(^<15>N及び^<13>C)された光化学系IIコア標品(ラン藻Synechocystis sp.PCC6803)の光誘起赤外吸収差スペクトルを測定し、Mhクラスターの骨格振動もしくはMnと酸素配位子間の相互作用に由来するバンドを特定した。また、低波数領域におけるアミノ酸配位子の炭素及び酸素に由来するバンドを特定した。 2.Mnクラスターの推定上の配位子と考えられているD1タンパク質のC末端アラニン(D1-Ala344)を構造的に最も類似性の高いグリシンに変異させることにより、Mnクラスターの骨格構造やアミノ酸配位子の配位構造を反映する振動モードに顕著な変化が見られたことから、C末端アラニンがMnクラスターの配位子であることを強く示唆する結果が得られた。また、複数のカルボキシレートを有する金属キレーターがMnクラスターの配位構造を変形させることを明らかにした。 3.光合成酸素発生反応の4つの状態間遷移に対応する光誘起赤外吸収スペクトル(1700-800cm^<-1>)を測定した。得られたスペクトルに対する^<15>N及び^<13>C同位体置換の効果について分析し、アミノ酸配位子に由来する振動を特定した。この領域ではH_2^<18>O置換の効果が見られなかったため、650-350cm^<-1>の領域について各状態間遷移に対応するH_2^<18>O置換の効果を検証した。その結果、650-600cm^<-1>に全ての状態間遷移において同位体シフトが観測され、これらがMnクラスターによる水分解反応もしくは酸素発生反応に密接に関与するクラスターの骨格構造の変化を反映するものであることが示された。競争的資金
研究シーズ
■ 研究シーズ- 光合成細菌による近赤外光のエネルギー資源化シーズカテゴリ:エネルギー, 環境・農学, 自然科学一般研究キーワード:光合成細菌, 近赤外光, 光電変換, アップヒルエネルギー移動研究の背景と目的: 光合成細菌は近赤外光を光電変換する光捕集・電荷分離システム(LH1-RC: 光捕集1反応中心複合体)を備えており、低いエネルギーを高いエネルギーに変換するuphill型エネルギー移動により近赤外光をエネルギー源とした光—物質変換を営んでいます(図1)。本研究では、様々な光捕集特性を示す光合成細菌を用いてuphill型エネルギー移動の分子機構を解明し、近赤外光資源化の基盤創出を目指しています。研究内容: 光合成細菌の光LH1-RCでは、①光捕集アンテナLH1が低エネルギーの近赤外光を吸収し、②LH1から反応中心RCへエネルギー障壁を遡るuphill型エネルギー移動が起こり、③そのエネルギーをRCが電気エネルギーに変換することにより、近赤外光を資源化していると考えられますが、詳細な分子機構は不明です(図2)。 当研究グループでは、様々な環境に棲息する光合成細菌を取り扱っており、光吸収特性やエネルギーギャップが異なるLH1-RC複合体について、それらの構造と機能の関連性を分光学的ならびに構造生物学的な手法により詳しく調べています。 これまでの研究から、①に関して、色素とタンパク質の相互作用、色素分子の種類や配向性、金属イオンの存在などが重要であることが明らかになってきました。現在、②のuphill型エネルギー移動および③の高効率な光電変換のメカニズムを明らかにする研究を進めています。
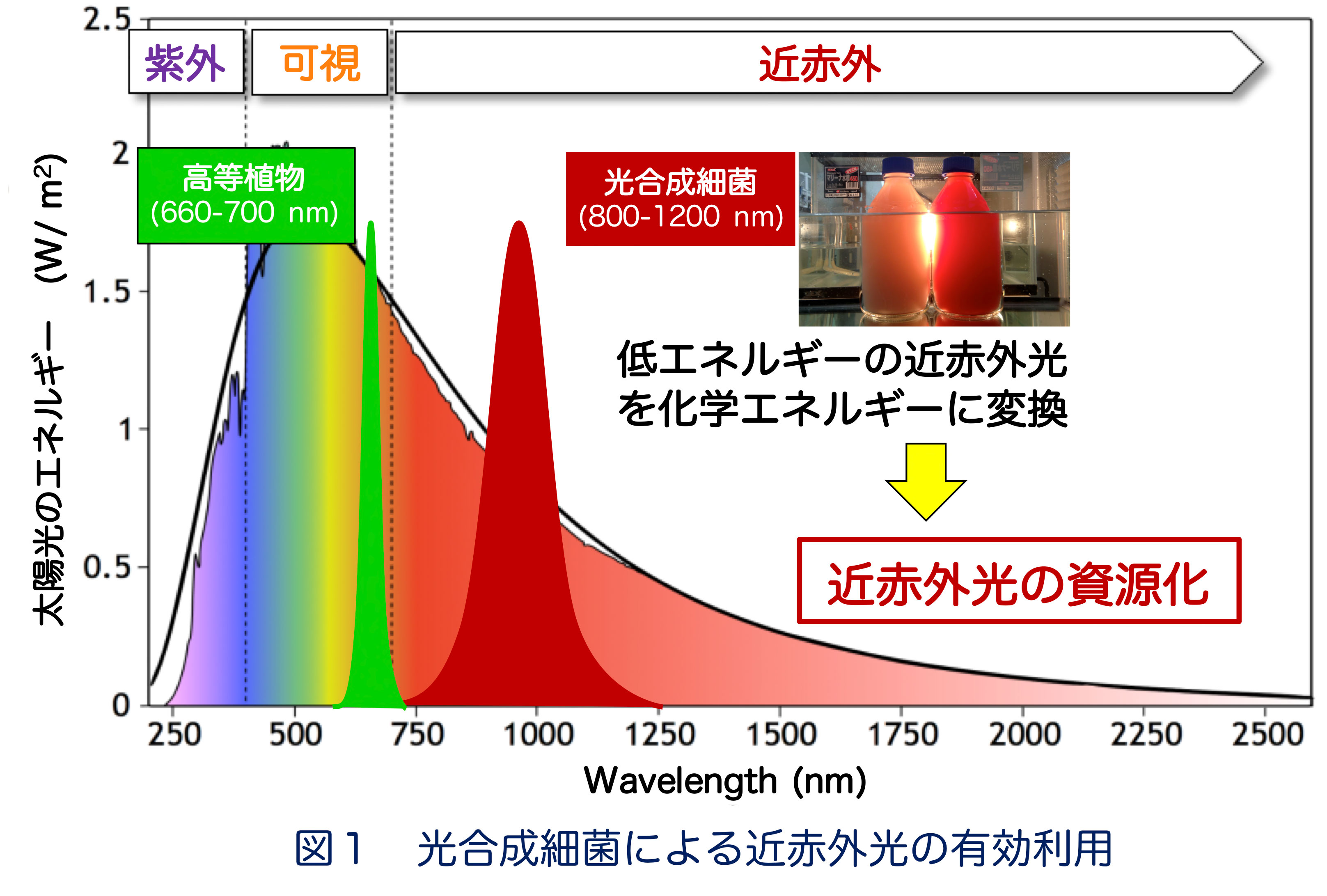
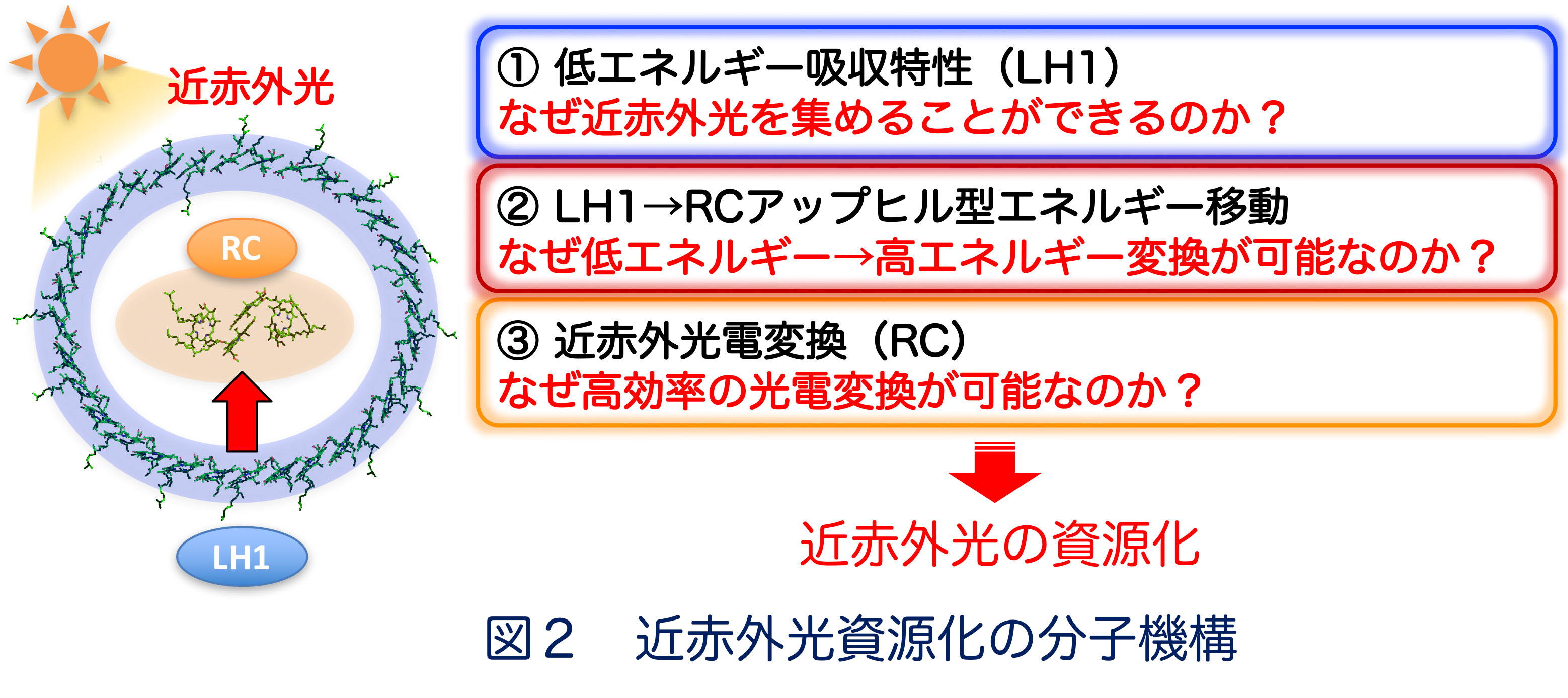 期待される効果や応用分野:図1に示すように、地上に降り注ぐ太陽光の約4割は近赤外光ですが、これらはエネルギーが低いため、未利用のエネルギー資源として浪費されています。光合成細菌はこの近赤外光を利用可能なエネルギーに変換しています。その分子機構の解明は、近赤外光応答型人工光合成系の構築に重要な知見となるものであり、新規エネルギー資源の基盤創出が期待されます。関係する業績:Y. Kimura et al. BBA 1862, 148473, 2021. Y. Kimura et al. Photosynth. Res. 148, 77, 2021. M. Imanishi et al. Biochemistry 58, 2844, 2019. K.V.P. Nagashima et al. PNAS 114, 10906, 2017.
期待される効果や応用分野:図1に示すように、地上に降り注ぐ太陽光の約4割は近赤外光ですが、これらはエネルギーが低いため、未利用のエネルギー資源として浪費されています。光合成細菌はこの近赤外光を利用可能なエネルギーに変換しています。その分子機構の解明は、近赤外光応答型人工光合成系の構築に重要な知見となるものであり、新規エネルギー資源の基盤創出が期待されます。関係する業績:Y. Kimura et al. BBA 1862, 148473, 2021. Y. Kimura et al. Photosynth. Res. 148, 77, 2021. M. Imanishi et al. Biochemistry 58, 2844, 2019. K.V.P. Nagashima et al. PNAS 114, 10906, 2017.

 people.kobe-u.ac.jp
people.kobe-u.ac.jp